usp class vi vs iso 10993
Evaluation and testing within a risk management process. ISO-10993 Standard USP Class VI Standard Other Industry Standards Page 2 Evaluation of Biocompatibility Page 6 Biomerics Polyurethane Resin Families Page 8 Quadrathane ALC Resin Results Page 9.

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing
Its possible that a USP Class VI material can also.

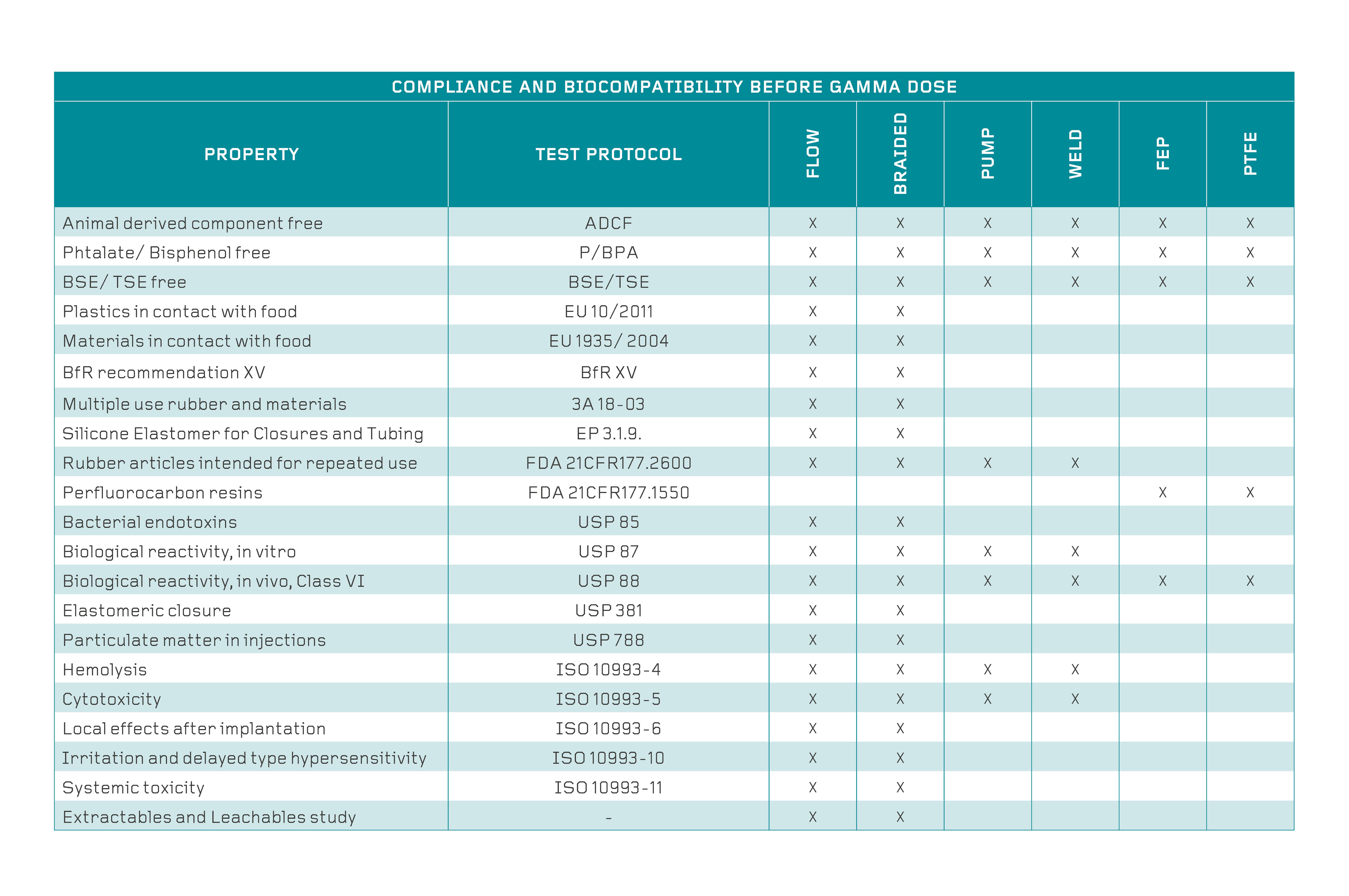
. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro ISO 10993-3 Ames Genotoxicity ISO 10993-11 Systemic Toxicity In-Vivo ISO 10993-4 Hemolysis Indirect European Pharmacopeia 329. These documents were preceded by the Tripartite agreement and is a part of the international harmonisation of the safe use evaluation of medical devices. By ensuring that a material is non-toxic and wont result in immunological rejection biocompatibility testing ensures that a rubber is safe for use with.
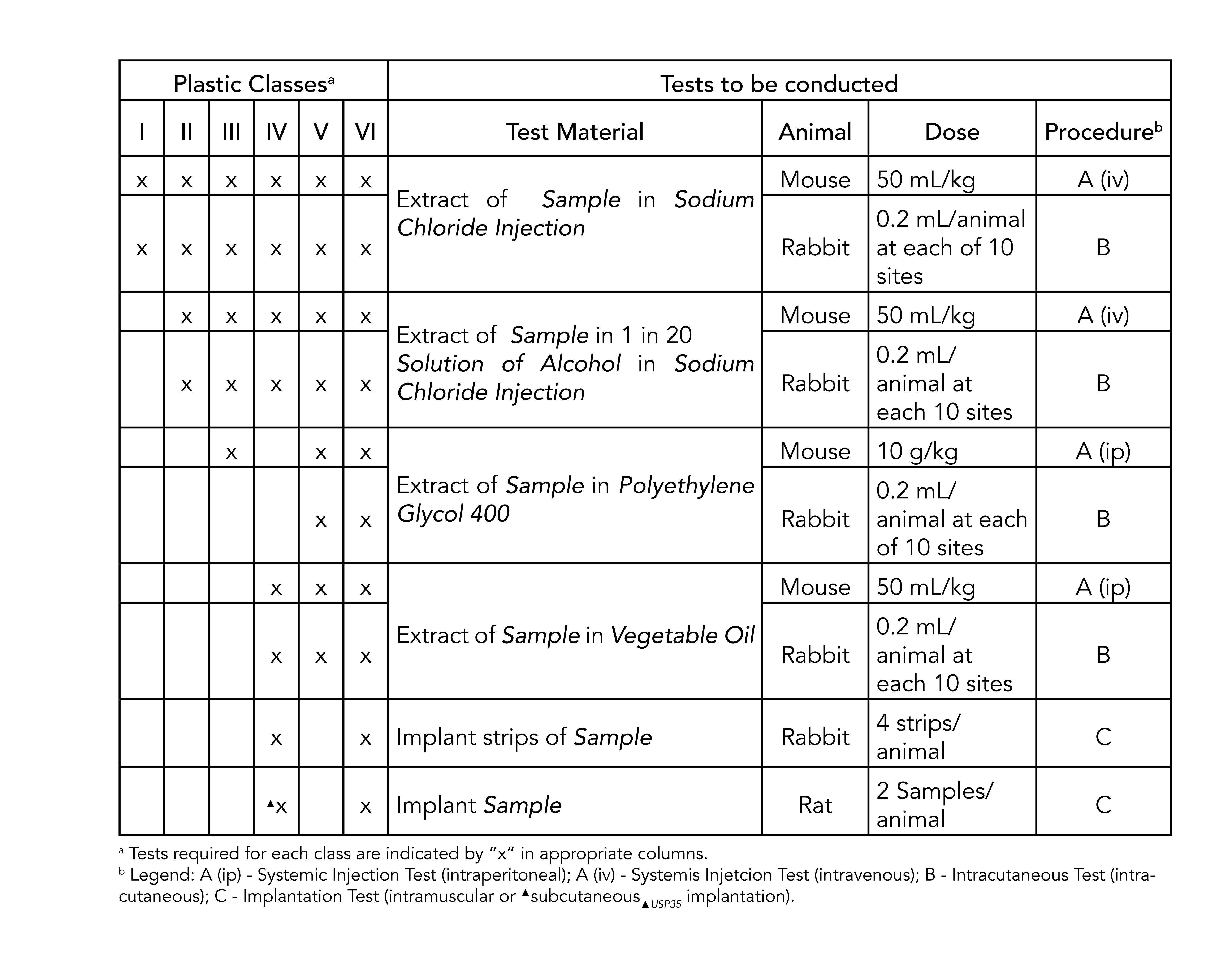
For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required. USP Class VI testing is conducted by producing an extract of the product with different extraction fluids such as polyethylene glycol and vegetable oil and injecting it in specimen rabbits and mice in vivo alive to observe the biological response to the extract. Testing to the highest ISO-10993 standards can add months of time and be very costly according to the Medical Device Testing Guide by Toxikon Inc.
May 1 2009. You might establish biocompatibility via making the device of a Recognized Consensus Standard material using a validated process that does not degrade that material or by ISO 10993 testing. Testing is commonly done as per USP which requires three types of.
Class VI and ISO 10993 are recommendations for testing based on the use of the final device. Biocompatibility testing biocompatible materials biocompatible rubber ISO 10993 medical molder medical molding medical silicones USP Class VI. ISO 10993 is a 20-part standard that evaluates the effects of medical device materials on the body.
Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10 or USP Class VI testing. Unlike other rubber standards theres no one standard that engineers use for an approval.
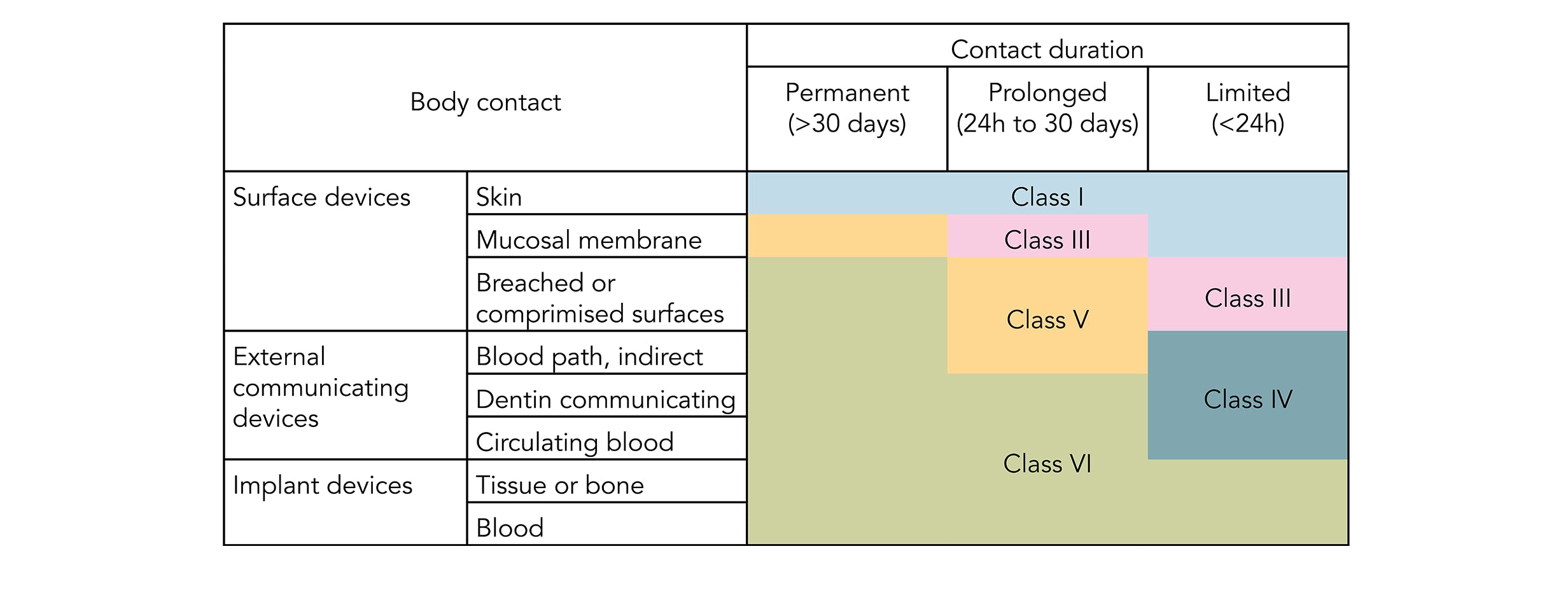
ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for shorter applications a USP Class VI or even a lower USP Class certification is often sufficient. USP Class VI vs. Then you need to understand the differences between ISO 10993 and USP Class VI and the nature of each standard.
For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. That said the lack of risk assessment in USP Class VI can be a problem. Medical Silicone Rubber Molding and Silicone Rubber Mold Materials.
Typical physical properties of C-Flex Property ASTM Method Formulations Value or Rating. We carry a wide range of materials from the worlds top medical resin suppliers including USP Class VI and ISO 10993 certified biocompatible resins with full FDA Master File support. Medical Molding and Biocompatibility Testing of Medical Devices Standards EN ISO 204172021 - Medical devices - Information to be Use of ISO 10993-1 Biological evaluation of medical ISO - ISO 18562-12017 - Biocompatibility evaluation of ISO -.
Testing for medical devices should be conducted using rinsingeluting and sampling techniques as described in ISO 10993-1 14 and ISO 10993-12 15 as. In an effort to standardize biocompatibility testing worldwide the International Standards Organization ISO developed ISO 10993. A more rigorous standard for the biological evaluation of medical devices is ISO-10993.
Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI. USP class VI versus ISO 10993 USP class qualification was a key method for establishing material biocompatibility at least as far back as 1976 until the 1987 adoption of the Tripartitite Agreement. Depending on the devices application and how the cable components will interact with the patient this higher level testing may not be needed.
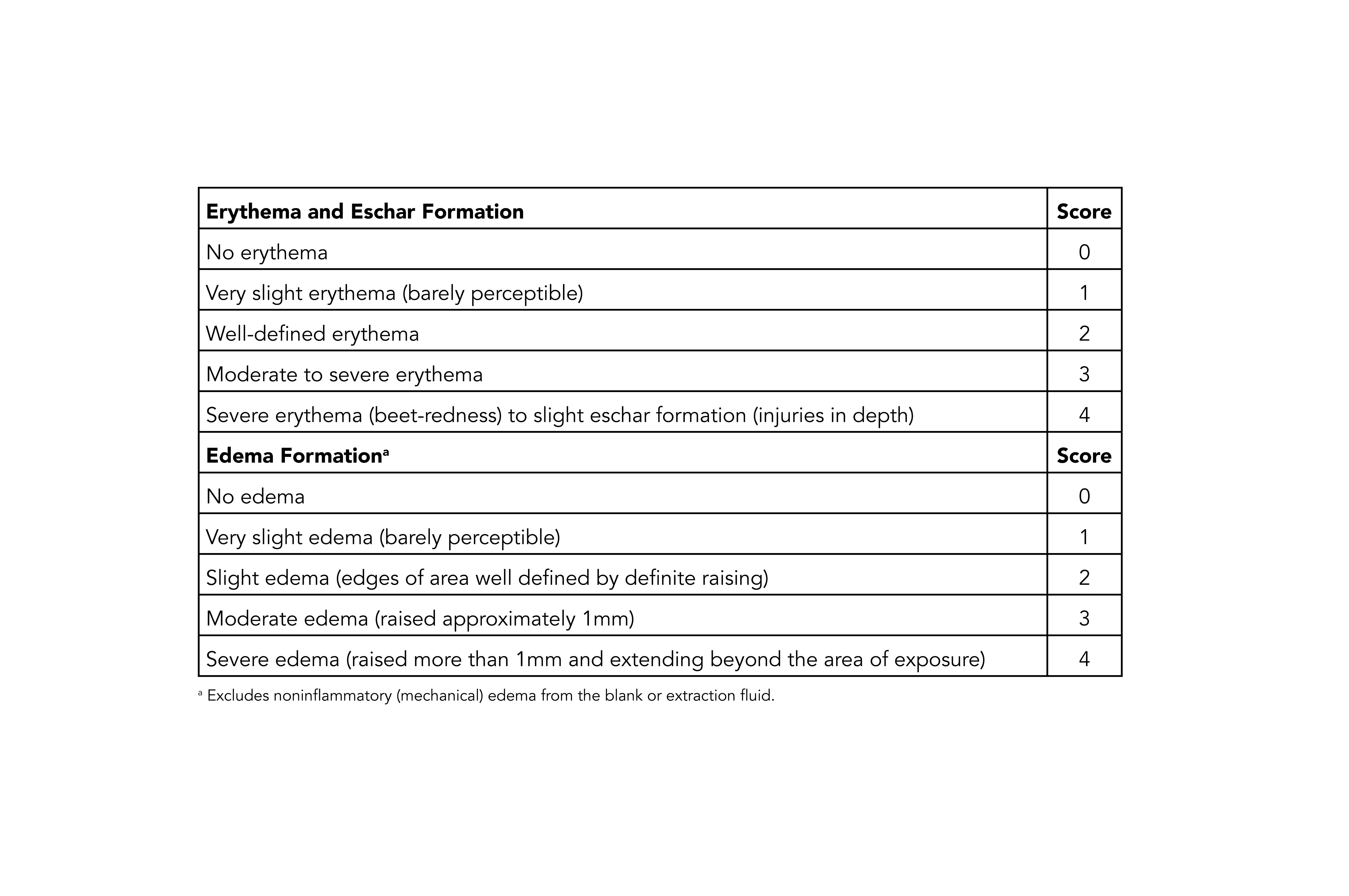
ISO-10993 is a standard that utilizes systemic toxicity and intracutaneous reactivity testing. USP Class VI testing alternatively can be. Tripartite introduced the first expectations of biocompatibility testing specifically focused on device-related material.
If yes to the first question then USP Class VI is not a relevant qualification for it. Take an ASTM D2000 call out. Biological evaluation of medical devices Part 1.
For the purpose of the ISO 10993 family of standards biocompatibility is defined as the ability of a. Most applications are fairly benign to elastomers. USP Class VI demands an intracutaneous irritation test.
At Venair all our tubing has a Validation Guide upon request as. Typically the terms USP Class VI or ISO 10993 materials are used. ISO 10993 - What are the differences.
The ISO 10993 set entails a series of standards for evaluating the biocompatibility of medical devices to manage biological risk. Depending on the devices use the sterilization process might. Biocompatibility Information for Materials.
USP Class VI. USP Class VI vs. A number of our plastic materials are ISO-10993 or USP Class VI capable.
USP Class VI Regiment Irritation Systemic Injection Implantation 1 week Biological Evaluation Plan BEP. The first part of the ISO 10993 standard Biological Evaluation of Medical Devices. While it is possible a USP Class VI material could also be ISO 10993 compliant its not a given and USP Class VI alone is not sufficient for adherence to ISO 10993.
USP class qualification was a key method for establishing material biocompatibility at least as far back as 1976 until the 1987 adoption of the Tripartitite Agreement. Our portfolio approach offers the most expansive selection of medical resin materials in the industry balancing performance cost. USP class VI versus ISO 10993.
However Class VI also requires subacute toxicity and implantation effects which many ISO 10993 categories do not. So does ISO 10993. A rubber compound has set physical parameters it needs to meet.
Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. That being said if you cant get an ISO 10993 compliant material often because the material simply hasnt been tested using a USP Class VI material is a less risky option.
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News
Biocompatibility Of Plastics Zeus

What Is Iso 10993 How Is It Different From Usp Class Vi Ppt Download

Material Selection Medical Injection Molding Xcentric Mold

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Usp Class Vi Foster Corporation

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group
E3609 70 Iso 10993 5 10 Compliant Epdm O Rings Parker O Rings O Ring Products United Seal

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com